

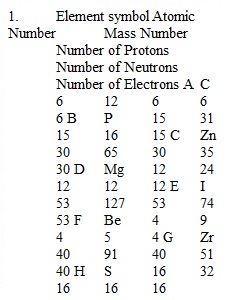
Q Unit 1-2: Atoms and Electronic Structure HW Assignment Be sure to use correct SF and units in all of your answers which involve calculations. SHOW ALL WORK: you will NOT receive credit if there is no work shown for calculations. 1. Complete the following table for the following neutral elements. Element symbol Atomic Number Mass Number Number of Protons Number of Neutrons Number of Electrons How many protons, neutrons and electrons are contained in each neutral element? 3. How many electrons do each of the following charged elements have? (Helps to determine proton number 1st) 4. Write the long version for the ground state electron configuration for each of the following elements. 5. Write out the noble gas (abbreviated) electron configuration for each of the following elements. 6. Draw and fill in an orbital diagram for the following elements. (boxes and arrows) ONLY complete a diagram for the valence electrons-those written in the HIGHEST ENERGY LEVEL. 7. Based on your orbital diagrams in the previous question, which elements are diamagnetic and which are paramagnetic? (list a, b, c, d & e)...................
View Related Questions